UPDATE: There also is a German version of this article available on my German blog: Leiterplatten mit Salzsäure und Wasserstoffperoxid ätzen
Introduction
Using a combination of hydrochloric acid (HCl) and hydrogen peroxide H2O2 in itself is nothing new. When I recently rediscovered this method (because I was out of sodium persulfate), I was amazed by the quality and sharpness of the resulting traces and pads. I shared my amazement on Twitter [1]. To my surprise, the responses were extremely divided. Many people confirmed that they have been using the same etchant for a long time and had great success with it. Others proclaimed it was a terrible etchant, extremely dangerous and poor controllability. As always, the devil is in the details. The group confirming the high reliability of hydrochloric acid and hydrogen peroxide as an etchant use – just like me – highly diluted solutions. Just like the common saying “it is the dose that makes the poison” goes, for use as an etchant it is absolutely essential to use proper concentration levels.
Etchant Preparation
For 1 litre of etchant the following ingredients are used:
- 700 ml distilled water
- 200 ml 30-35 % hydrochloric acid (HCl)
- 100 ml 12 % Hydrogen Peroxide (H2O2)
All ingredients are added together in a beaker. Water is added first, then the hydrochloric acid, lastly the hydrogen peroxide.
Concentrated hydrochloric acid, also called muriatic acid, does fume a lot and the fumes are quite unpleasant to breathe in. The fumes are also highly corrosive to anything near it. So in order to mitigate the possible hazards, and to simplify the preparation of the etching solution, I prepared an 8 % hydrochloric acid solution in a well ventilated area. Additionally, I prepared a 12 % hydrogen peroxide solution. Both are safe for storage and use in a regular indoor lab. The concentrations were chosen to further simplify the preparation of the etchant: 9 parts of the 8 % HCl and 1 part of the 12 % H2O2 solution are added together to yield 10 parts of ready to use etchant solution. For example, adding 90 ml of the 8 % HCl and 10 ml of the 12 % H2O2 solution yield 100 ml of etching solution.
Another option, especially in countries where 12 % hydrogen peroxide is hard to come by, would be to use 3 % H2O2 and premade 14 % hydrochloric acid. In that case, the mixture ratio is further simplified to 1:1. For instance, 500 ml of 14 % HCl and 500 ml of 3 % hydrochloric acid would yield 1 litre of etchant.
It should be noted that hydrogen peroxide likes to decompose when exposed to light. Therefore, the H2O2 stock solution should be kept in an amber glass bottle, or at least be kept away from (natural) light sources.
Hazard of Chlorine gas production
A common warning is, that this method produces extremely dangerous and toxic chlorine gas. While chlorine is always produced (Eq. 1 and 2), it’s release as chlorine gas does depend strongly on the concentration of the HCl and H2O2 used [2]. If the concentrations are kept below a certain threshold, the chlorine is staying in solution and is eventually consumed in another reaction mechanism (Eq. 3).
(1) ![]()
(2) ![]()
(3) ![]()
So how much is too much? To quote directly from a research paper titled “Oxidation of hydrogen chloride with hydrogen peroxide in aqueous solution”:
The oxidation of hydrogen chloride with hydrogen peroxide at common temperature with the evolution of chlorine into the gas phase occurs at a hydrogen chloride concentration in a solution exceeding the threshold value (5.2 M).
A 5.2 M concentration of HCl corresponds roughly to 16.1 %. The concentration used in the etchant recommended in this article is about 2.27 M, less than half of the critical threshold level!
Etching Process
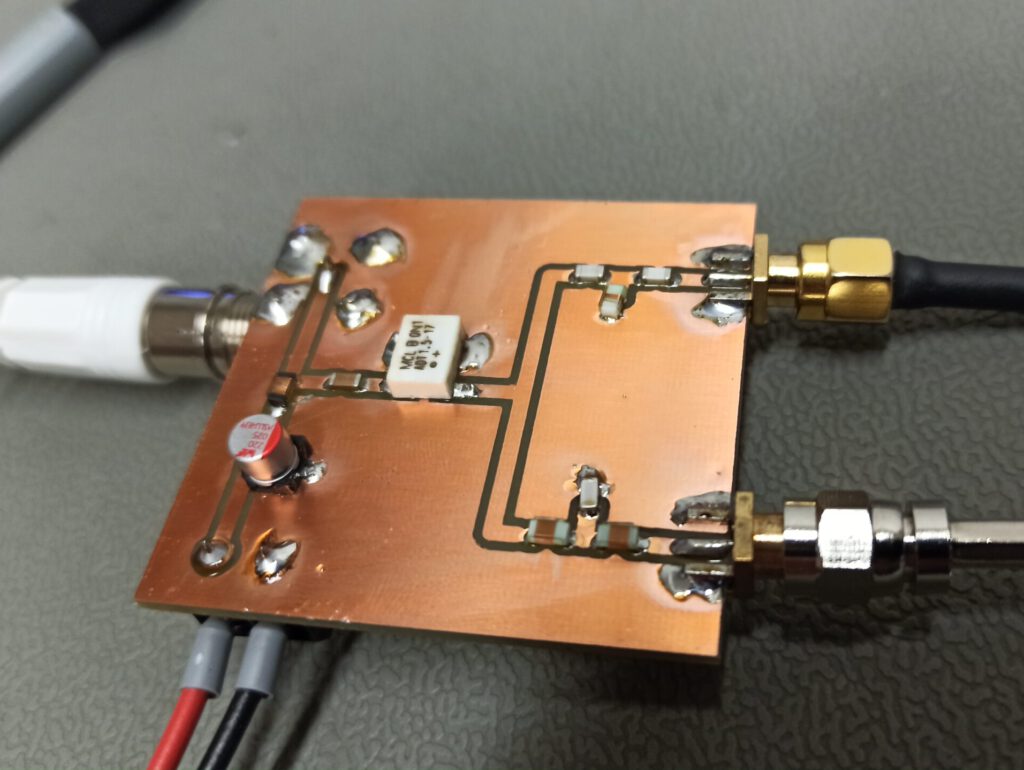
When ready to etch a PCB, I prepare the necessary amount of etchant as describeb before. A glass beaker is used as the container for the etching process. The PCB is completely submersed in the etchant. For a copper thickness of 35 µm (1 oz) the etching time is about 17 minutes at room temperature. After the etching has completed, remove the PCB from the etchant and rinse the PCB with water.
Conclusions
The results are quite satisfactorily. The quality of the traces in regards of sharpness, lack of overetching is outstanding. While standard precautions should be in place when working with chemicals, I can involuntarily state that even a few drops of the etchant won’t eat your flesh anytime soon. Which is no surprise at these high levels of dilution.
In the beginning of the etching process, the copper is attacked and copper chloride is formed. After a while, the etching process will follow the same chemical principles of using Copper(II) chloride directly [3]. Having a bit of cupric chloride in the etching solution to begin with might help the etching process. In any case, due to it’s regenerative nature, the etching solution should last for a long time. That said, I have never attempted to store used etchant and always prepare a fresh solution when I need it.
YouTube Video
Links and Sources:
[1] Twitter Post, Baltic Lab: https://twitter.com/BalticLabEE/status/1687521760386670592
[2] Oxidation of hydrogen chloride with hydrogen peroxide in aqueous solution, Skudaev, V. and Solomonov, A. and Morozovskii, A. and Isakov, N.: https://link.springer.com/article/10.1134/S1070427208010035
[3] Etching with Air Regenerated Acid Cupric Chloride, By Adam Seychell: http://techref.massmind.org/Techref/pcb/etch/cucl2.htm
Westerhold, S. (2023), "Etching PCBs using hydrochloric acid and hydrogen peroxide". Baltic Lab High Frequency Projects Blog. ISSN (Online): 2751-8140., https://baltic-lab.com/2023/08/etching-pcbs-using-hydrochloric-acid-and-hydrogen-peroxide/, (accessed: July 8, 2025).
- WebP-Images without Plugin - January 14, 2025
- Firewall Rules with (dynamic) DNS Hostname - January 14, 2025
- Restoring proxied visitor IPs from Cloudflare - December 26, 2024


Zachar Laskewicz
Thanks for this! I have my HCl and Hydrogen Peroxide and am planning to use these two to etch copper plated board and steel plates. Thanks to your information I’ve got a basis for mixing the acid and the peroxide at safe levels. If I understand it correctly, the 8 percent solution is made by combining 700 ml of water with 200ml of 30-35 percent hydrochloric acid? My HCl is 23 percent but I’ll work out how much will get it to eight percent before adding the peroxide. I was using the HCl to degalvanize the steel before the etching, so I had enough of it to try your method. If it doesn’t work I’ll play around with the solutions until it does. So wonderful that you shared this information! Great!!